
However, as frequently happens, the success did not survive closer examination and the advances in knowledge after Bohr’s work. It used an amalgam of classical physics and what has become known as the ‘old quantum theory’. This theory represented a significant advance on classical physics. We will look at the elements of Bohr’s argument in Subsection 2.1. Transitions by the electron between these levels, according to Bohr’s quantum theory of the atom, correctly predicted the wavelengths of the spectral lines.
He started by looking at the electron in a circular orbit about the proton and derived an expression for the corresponding energy levels. Application to the hydrogen atom was first tried by Niels Bohr in 1913. Radiation of frequency f transfers its energy in quanta of amount hf. Initially, the theory was applied to radiation. The arrival of the quantum theory at the beginning of the twentieth century offered a new approach.
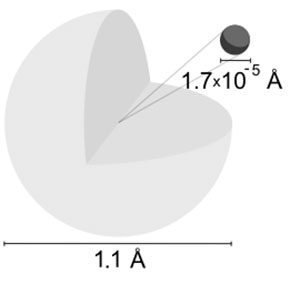
Classical theory, therefore, not only fails to predict the characteristic line spectra, it suggests that the atom is inherently unstable! The loss of energy by the electron will cause it to spiral in towards the proton and eventually crash into it within a tiny fraction of a second. Indeed, the theory predicts that an electron in orbit about the proton will continuously emit electromagnetic radiation. However, even after the discovery of the nuclear atom by Rutherford in 1912, the classical physics of the nineteenth century, when applied to the electron in the atom, could not account for the hydrogen spectrum at all. This was before the discovery of the electron, so no theory could be put forward to explain the simple formulae. Nineteenth century physicists, starting with Balmer, had found simple formulae that gave the wavelengths of the observed spectral lines from hydrogen. In this module, we will look at the attempts that have been made to understand the structure of the hydrogen atom – a structure that leads to a typical line spectrum. It is therefore not surprising that it has been the test–bed for new theories. It has only one electron and the nucleus is a proton. They used to consider electrons spinning in orbit around a nucleus as a charged particle moving in an electric field, but there was no explanation for the fact that the electron would spiral into the nucleus.The hydrogen atom is the simplest atom. The previously proposed planetary models of the atom also had some problems. Previously the Rydberg formula was known only experimentally, but theoretically, it was not proved, the Bohr model introduced the theoretical concept successfully. The model was able to successfully explain the concept of the Rydberg formula for the spectral emission lines of atomic hydrogen.
HYDROGEN ATOM MODEL ANDROID
Android App Development with Kotlin(Live).
HYDROGEN ATOM MODEL FULL


 0 kommentar(er)
0 kommentar(er)
